Surgihoney: super honey?
Ian Staples, Co-founder of Surgihoney
Introduction
Nature has evolved defence mechanisms to deal with microbial infection; for example, active oxygen plays a key role in plant and animal tissue microbial natural defence mechanisms. Where hydrogen peroxide is produced in nature, the enzyme catalase is always present, acting as a buffer to prevent tissue damage that may arise from high active oxygen levels. This action is the key to the understanding why nectar, the key base of honey, doesn’t ferment despite being comprised of fructose, glucose, sucrose, carbohydrates, amino acids and water [1].
Although honey has been used for wound/infection management for millennia, it was only ‘rediscovered’ as a topical antibacterial agent in 1892 [2]. A number of honeys, principally manuka honey and its derivative dressings, are accredited as medical grade honeys but have only developed to be wound care products. Development has been seriously limited by two factors:
- supply; it depends on nectar derived from a particular shrub in New Zealand, Leptospermum scoparium, which is produced in very limited amounts
- in vitro, the antimicrobial characteristics of a comparator honey dressing were partial - 30 per cent of bacteria taken from human tissue were not affected [Table 1]
Bees and nectar
Honey bees collect nectar as a carbohydrate source during summer and store it in the hive to both dry it (the moisture content needs to be below circa 18 per cent to prevent fermentation by the yeast) and for food during winter. The nectar is a weak solution of simple sugars and contains variable but often very high levels of yeast cells. However, it was observed that nectar does not ferment, even when the combination of high levels of moisture, simple sugars, warmth and yeast should lead to very rapid fermentation. Put simply, after five million years, the yeast microbe has not succeeded into evolving resistance to the antimicrobial properties of nectar.
This observation was then linked to the observation that nectars produce hydrogen peroxide at low levels. Hydrogen peroxide and the formation of reactive oxygen are increasingly linked to organic/natural systems’ antimicrobial defence mechanisms. Honey has no free water so cannot ferment, and for most honeys, this hydrogen peroxide production capability is lost. In the process of millions of years of evolutionary development, honey just did not need this protection.
These two observations led the research team at Surgihoney (Healing Honey International, UK) to create a propriety managed and controlled process that engineered this characteristic into honey (Box 1). Surgihoney can be produced from any pure honey from any floral source; its production is not limited as there are 100’s of thousands of tons of honey produced globally that meet this criteria.
Box 1: Surgihoney
Surgihoney is a CE approved medical device, bioengineered to be effective against antibiotic resistant species or stains such as MRSA, E.coli and Pseudomonas aeruginosa [Table 1]. While all honeys have antibacterial properties, the bioengineered honey is designed to contain even more of the agents that prevent bacterial growth. The ability to produce low levels of hydrogen peroxide in a wound over an extended period of time is why Surgihoney has such high potency against every microbe tested and that have been taken from human wound tissue [Table 1]. While all honeys have antibacterial properties, Surgihoney is designed to contain more of the agents that prevent bacterial growth. Its ability to produce low levels of hydrogen peroxide in a wound over an extended period of time is why Surgihoney has such high potency against every microbe tested from human wound tissue.
At the Royal Hampshire County Hospital, it has been shown to kill MRSA, and halve post-operative Caesarean section infection rates [3]. Surgihoney has also been used on complex infected surgical wounds, pressure ulcers and leg ulcers; none of the wounds treated deteriorated, and the majority have showed an improvement
Antimicrobial effect
Surgihoney is active against both gram-positive and gram-negative bacterial wound isolates [4]. Extensive in vitro work has demonstrated the broad spectrum antimicrobial characteristics of Surgihoney. In vitro studies undertaken at the Royal Hampshire Hospital in Winchester, compared the efficacy of both a commercialy available honey dressing (dresssing A) and Surgihoney. This simple analysis demonstrated the consistency and efficacy of Surgihoney against all the pathogenic bacteria tested [5]. In 3 out of 9 (30 per cent) of the bacterial groups, dressing A had no effect against the pathogen (Table 1).
Clinical observations
Supporting this in vitro work, Surgihoney has been used to control chronic infections in complex acute and chronic wounds such as amputations, pressure ulceration, leg and foot ulceration and caesarean section wounds[4] [6]. All wounds treated have shown a rapid reduction in infection levels and then progression to healing, as evidenced by the three case reports below.
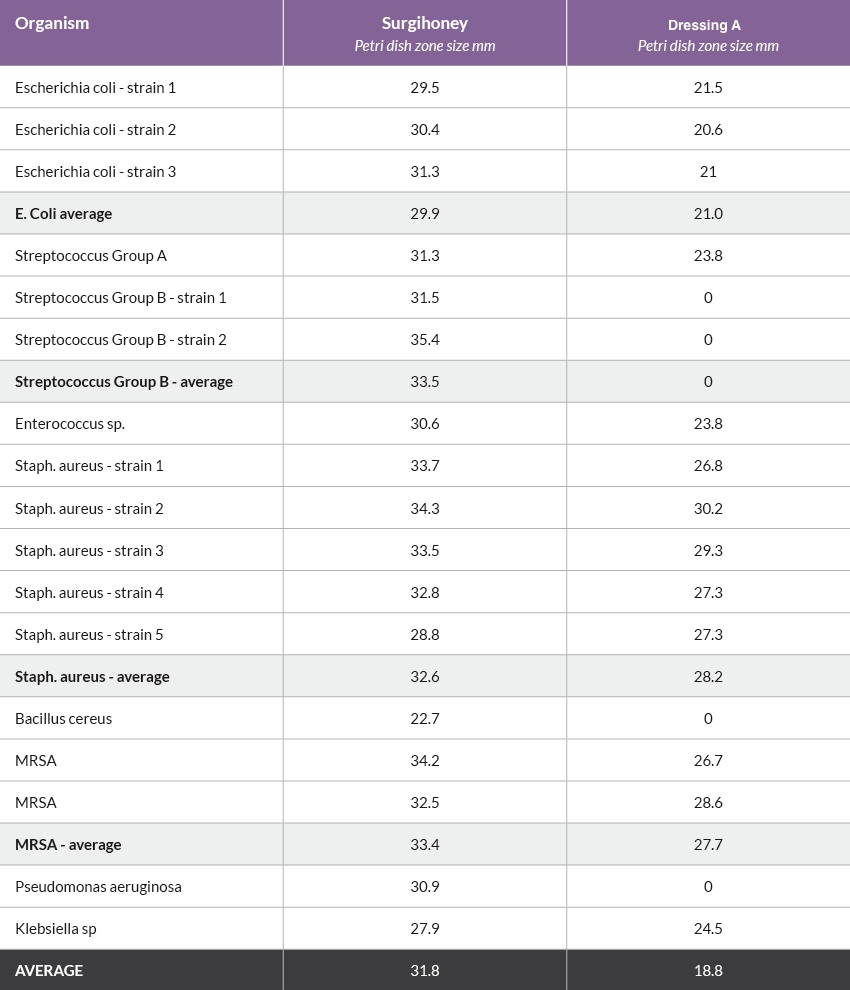
Case 1: Baby with MRSA infected surgical wound
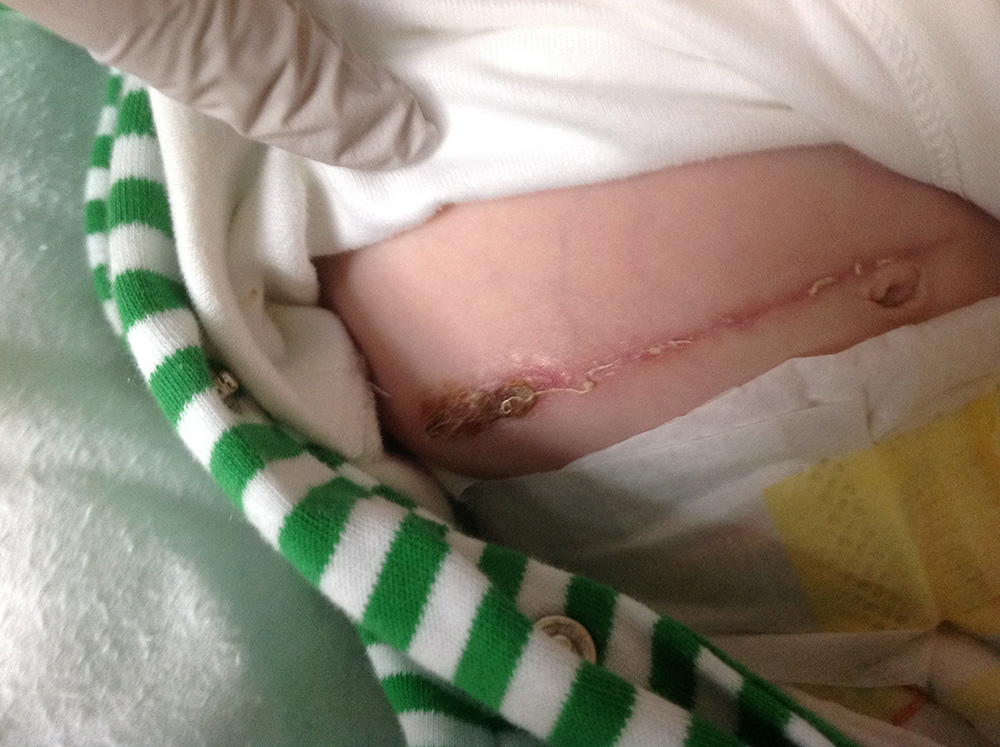
This baby underwent surgery for a twisted bowel; necrotic bowel was excised and the healthy ends were anastamosed. Post-operatively the baby developed inflammation in the lateral portion of the wound and methicillin resistant Staphylococcus aureus (MRSA) was isolated (Figure 1). This did not warrant antibiotics, so Surgihoney was started.
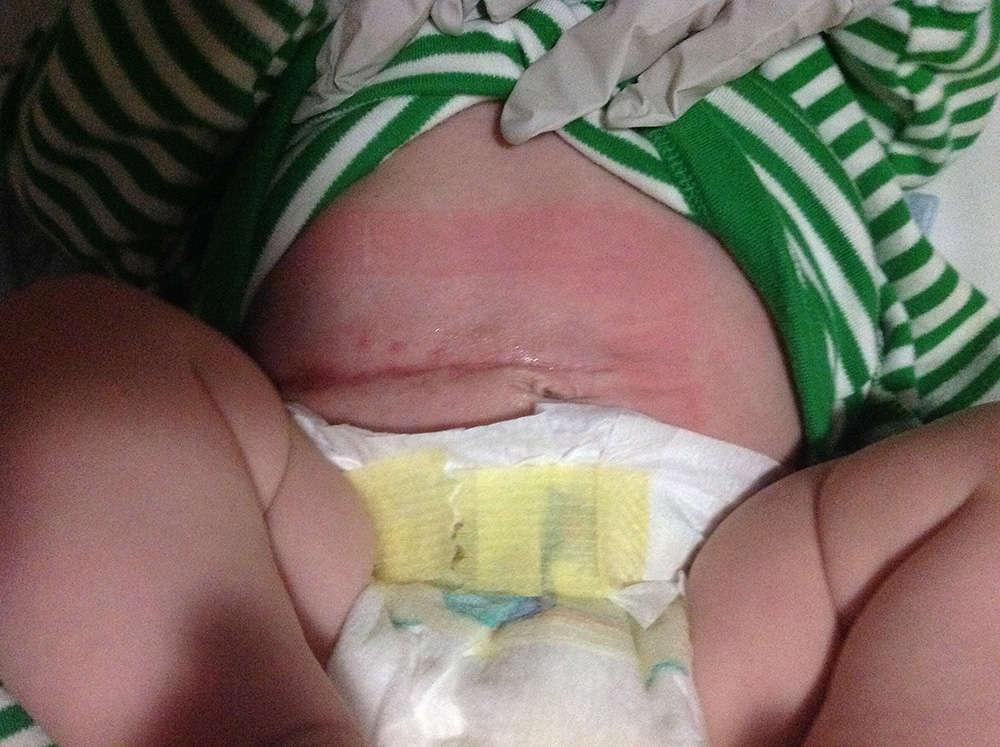
By day four the clinician reported wound improvement, but by day 11, the wound was still inflamed with MRSA present. The clinical team considered discontinuing Surgihoney and using an alternative topical agent, possibly with systemic antibiotics. However on closer investigation, it was found from day three, Medihoney was being applied rather than Surgihoney. Error corrected, Surgihoney was applied daily from days 12 to 19, by which time the wound had completely healed and MRSA was no longer detected (Figure 2). Surgihoney was continued for a few more days as a cautionary step.
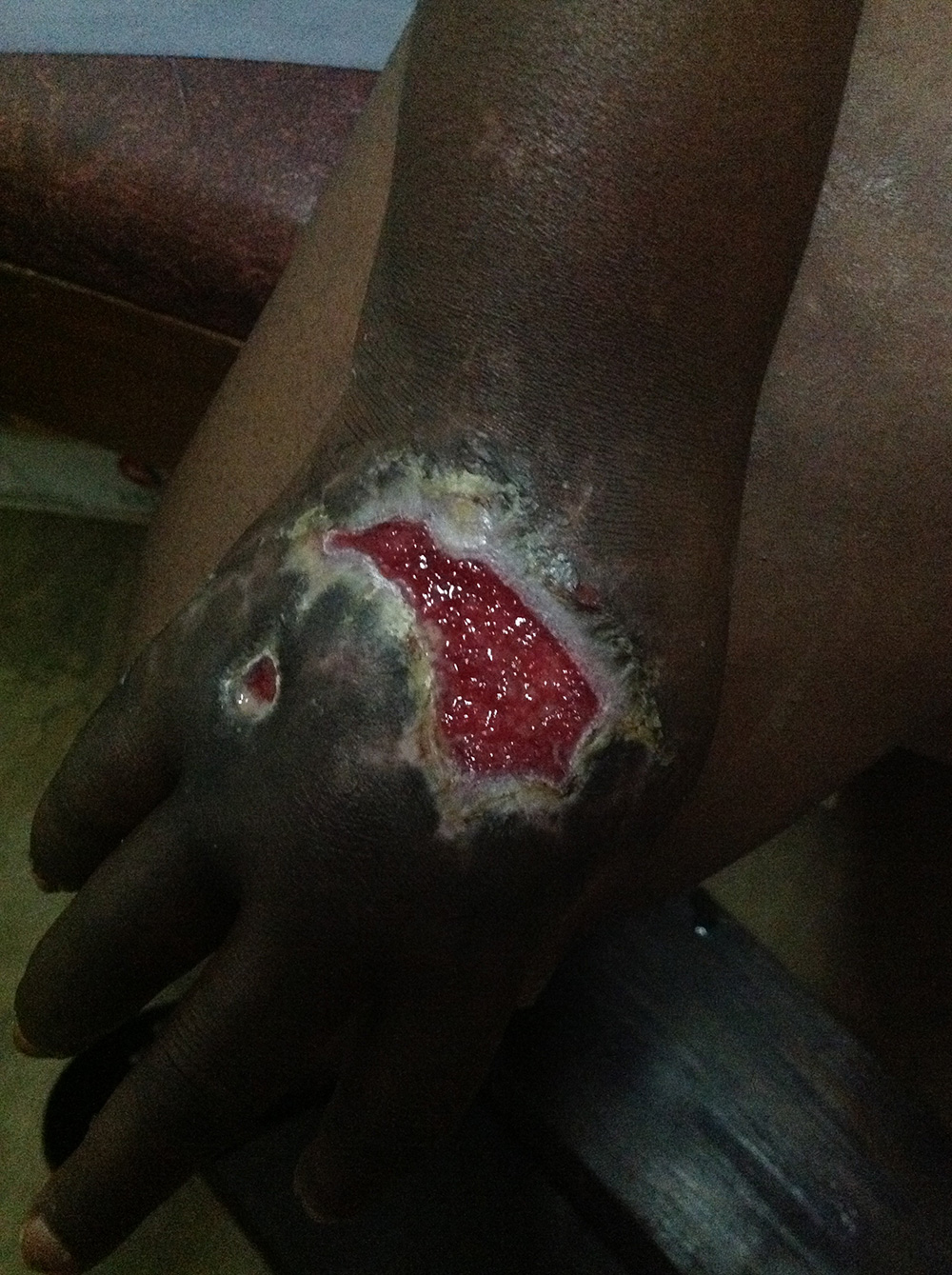
Case 2: Traumatic infected hand wound
The Surgihoney team were interested in assessing the effectiveness of Surgihoney in the much tougher clinical conditions of sub-Sahara. The wanted a product that could become a global wound care solution in the rich regions of the world with the very best clinical care to the poorest and least resourced regions where many factors made wound repair very difficult.
This young woman in Uganda had suffered a traumatic wound a month prior arriving at the hospital. It was deep, painful and heavily infected and amputation was a possibility (Figure 3). One principle attraction of Surgihoney is the simplicity of its use. Surgihoney was applied every other day on a simple dry gauze dressing.
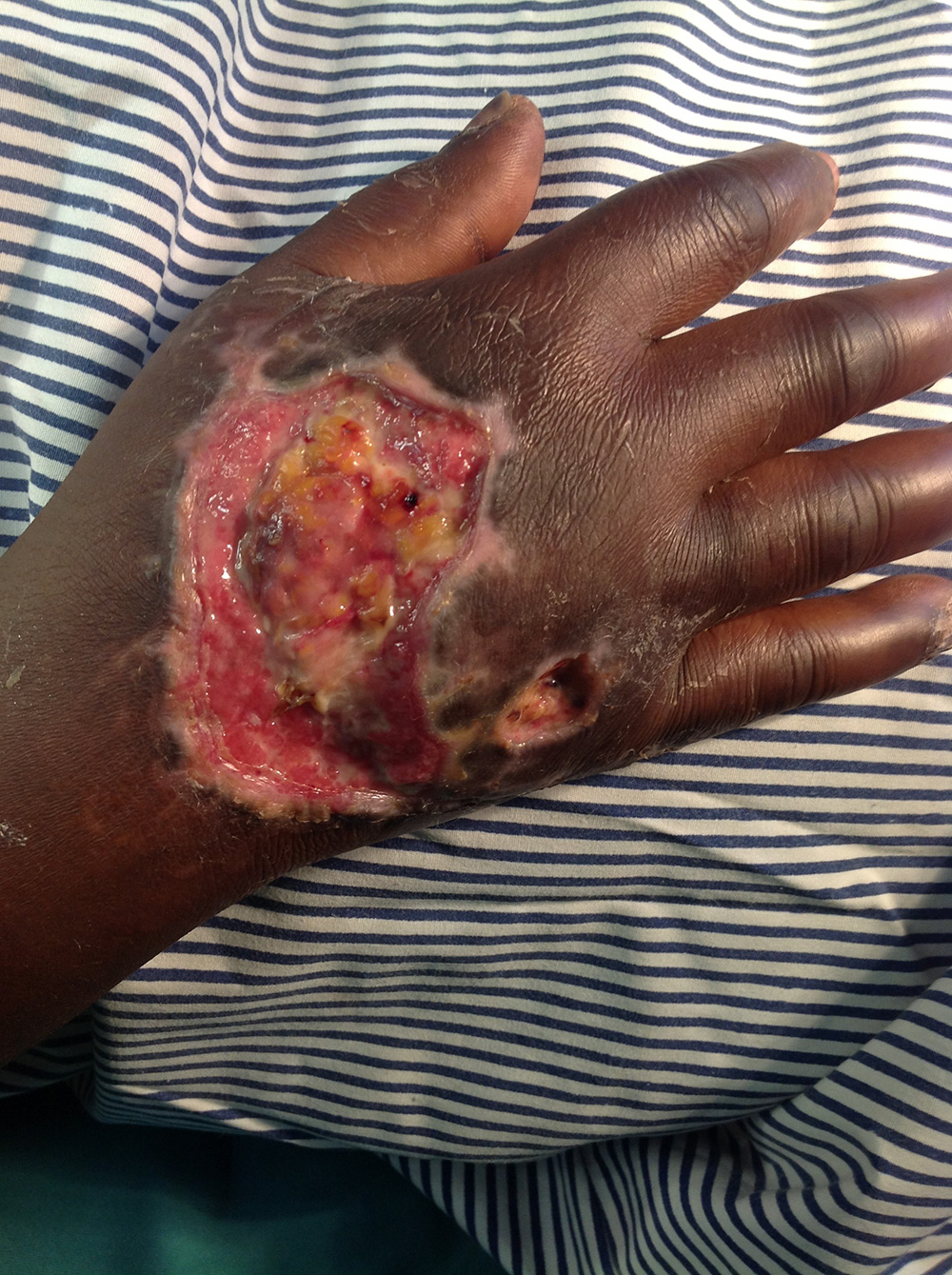
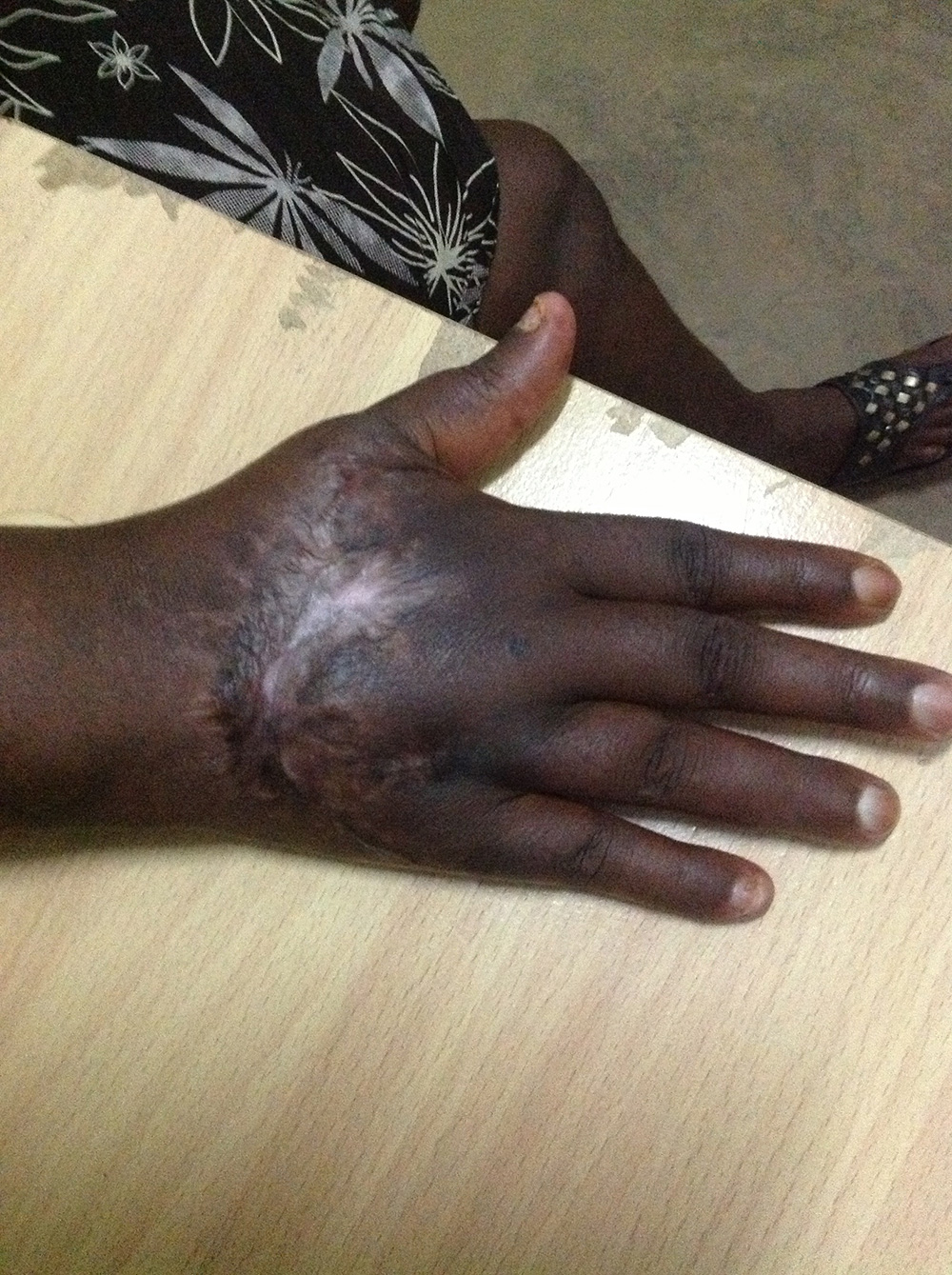
By day 12 , a significant improvement in the wound condition was recorded; the bacterial infection was eliminated and healthy new tissue was developing (Figure 4) . No antibiotics had been used, although Diclofenac was given for pain.
As healing progressed, Surgihoney was applied every three days to prevent re-infection and to provide the therapeutic stimulation to accelerate healing. By day 64 the wound had healed (Figure 5). The young woman could return to a normal life rather than spending the rest of her life maimed and marginalised.
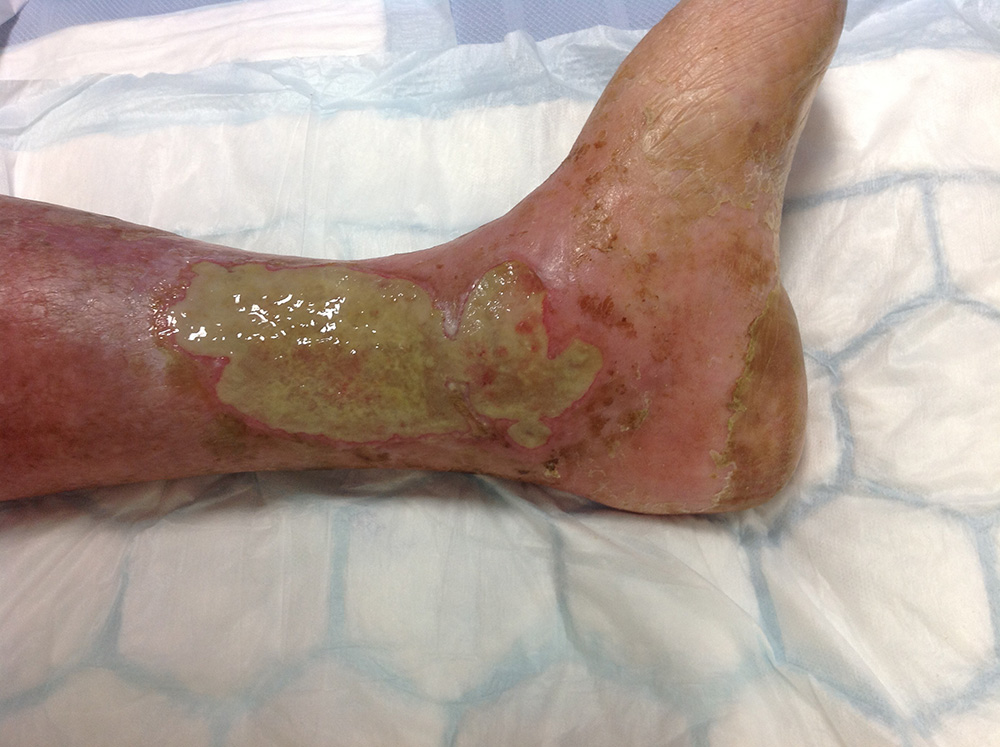
Case 3: Leg ulcer chronically infected by Pseudomonas aeruginosa
This elderly male had been a heavy smoker for years, and suffered from vascular disease and emphysema. He suffered from recurrent leg ulceration, this particular ulcer being present for well over six months and heavily infected with Coliform bacteria and Pseudomonas aeruginosa (Figure 6). The vascular team had tried bed rest and conventional treatment to no avail, and he was being considered for amputation.
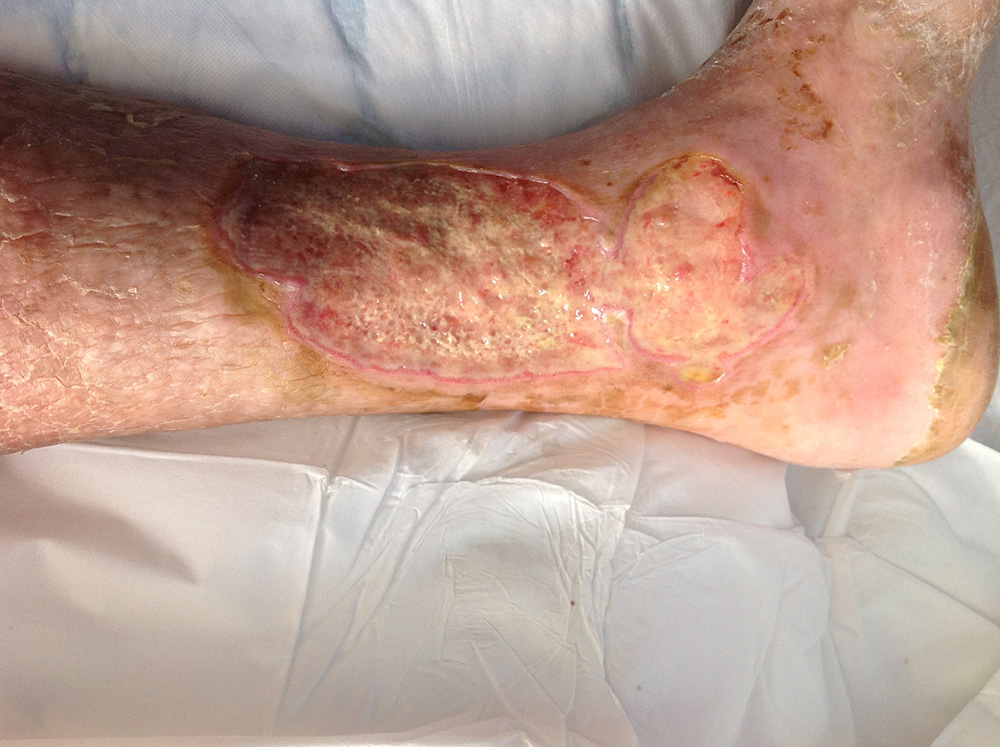
Surgihoney was applied and after four days there was a marked change in the visual appearance of the ulcer; the wound-bed changed from deep green to fluorescent lime, indicating that the Psuedomonas bacteria was dying as it was fluorescing. Seven days later, the ulcer had improved to the point that the patient was ready for discharge from the hospital. All the green slough had gone, the bacterial infection level had gone from critical to minimal. The wound bed was beginning to show healthy new tissue formation (Figure 7).
Discussion
The efficacy of manuka honey as a partial antimicrobial agent is due to the presence of a toxic product referred to as methylglyoxal (MGO). This product comes directly from the nectar of the manuka flower and passes unchanged into the honey; it is found in very high levels in this nectar source but not in other honeys. It could be postulated that if MGO was nature’s way of dealing with microbial infections, then it would be found in many other defence mechanism and in all honey to prevent to nectar fermenting, but it isn’t. In addition, part of the manuka testing protocol is to add large amounts of catalase to the product, leading to ‘non-peroxide activity’. However, this protocol has mislead the scientific world, as the catalase levels used are (in) many multiples more than found in any natural catalase/peroxide balance [7] . In Goth’s paper, normal human serum level of catalase were detailed at approximately 50.5 +/- 18.1 kU/L (approx 70kU/L), equating to 70 units per ml in undamaged tissue. The quantity used in the NZ protocol is 2 mg/ml solution of catalase from bovine liver (Sigma C9322, 2900 units/mg ), diluted into the honey 50/50, giving a final concentration of 2900 units per ml - 40 times more catalase than normal serum levels.” In other words the NZ protocol used catalase many times higher than would be found in natural tissue balance between the production of hydrogen peroxide and naturally occurring catalase.
Irrespective of this data, the consistent results of eliminating or reducing complex chronic infections in wounds using Surgihoney indicates very pragmatically that the presence of low levels of hydrogen peroxide in a wound and its efficacy at killing microbes is not damaged by the natural presence of catalase in the wound exudate.
As the Surgihoney development is not limited to a limited floral source it has the ability to be a global wound care solution in the richest parts of the world where the best clinical care is available, and for to the patient in the poorest Indian or African village.


























